ZnCuCl2 Cu ZnCl2. M g C l X 2 a q K X 2 S O X 4 a q F e N O X 3 X 2 a q S n C l X 4 a q C u N O X 3 X 2 a q F e B r X 3 a q K C l a q C u S O X 4 a q I would like to know why and how can I recognise when a reaction occurs or it does not.
Single Displacement Reaction Definition Examples Video Lesson Transcript Study Com
Some single replacement reactions will happen others will not.

. A chemical reaction in process Photo Credit. Isnt the halogen ion iodide more reactive than ceNO3-. Halogens can replace other halogens.
If there were no precipitate or undissociated product then you would end up so to speak with the same four species that you started with. Most reactive Li Rb K Ba Sr Na Replace an H in H2O to form metal. Zinc and Copper II Ion REDOX.
ABC B AC. This type of reaction has the general equation. A metal replaces another metal that is in solution.
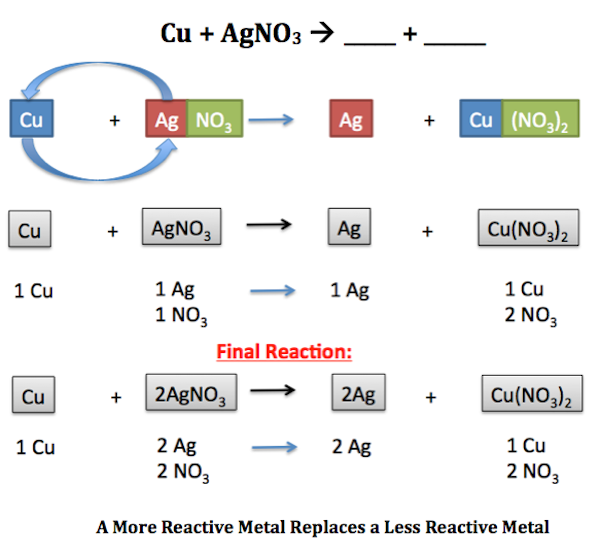
PREDICTING WHETHER A SINGLE REPLACEMENT REACTION WILL OCCUR. In order to determine if a single replacement reaction will occur you must use the Activity Series. When zinc metal is immersed in a solution of 01 M aqueous copper II sulfate solution copper metal plates out on the zinc.
Thus you have to understand the activity series. For example ce2KI PbNO32 - 2KNO3 PbI2. The periodic table or an activity series can help predict whether single-replacement reactions occur.
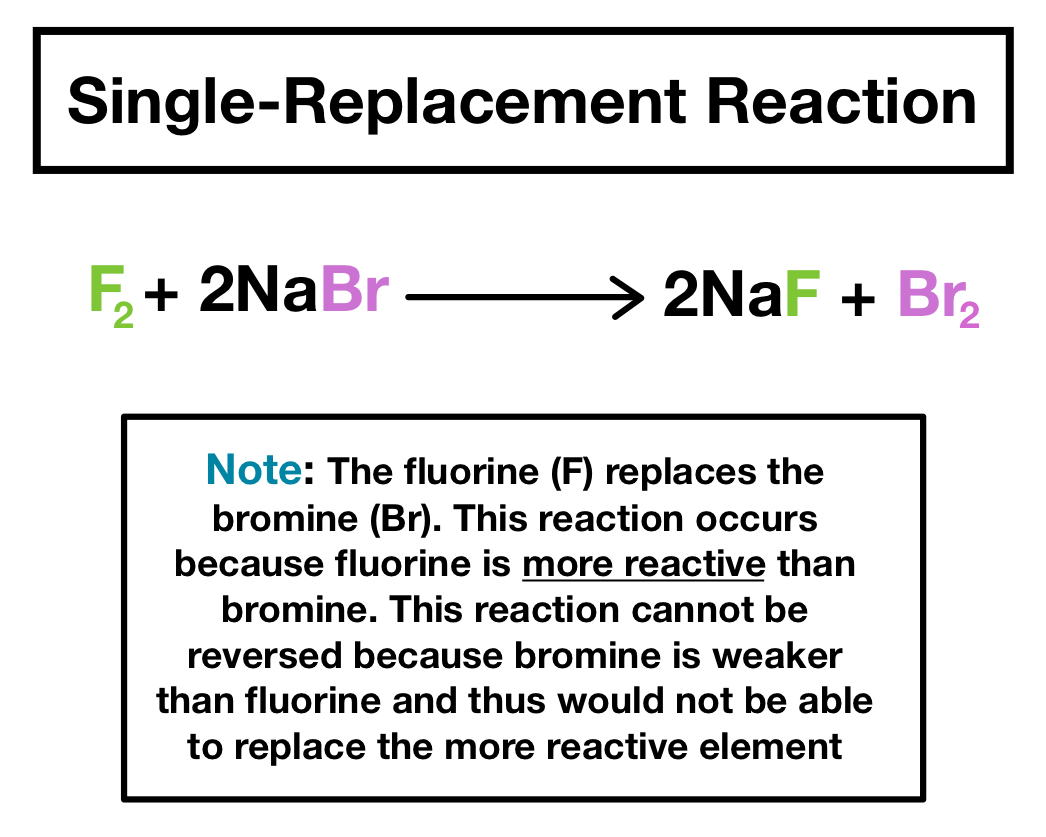
Zn CuCl_2 Cu ZnCl_2 A halogen replaces another halogen that is in solution. 3 Mgs 2 FeBr 3 aq 3 MgBr 2 aq 2 Fes. And are halogens and is a cation.
Show activity on this post. A dark coating of copper metal appears on the zinc within two minutes and when 45 minutes have elapsed there is a. So is there anything limiting double displacement reactions.
Single replacement reaction will occur. Single replacement reactions occur if the lone element can replace a similar element in a compound. A metal replaces another metal that is in solution.
There are two types of single replacement reactions. ShaiithShutterstock A replacement reaction is a type of chemical reaction in which one element replaces another in a compound. When an element replaces another element from a compound a single displacement reaction has occurred a more active element with replace a less active element from its compound.
The overall pattern of a double replacement reaction looks like this. Metals can replace less active metals. A BC B AC Example.
A single-displacement reaction occurs when an element replaces another element in a compound. Generally more reactive elements replace less reactive elements. Experiment 10 - Single Replacement Reactions A single replacement reaction is a type of oxidation-reduction reaction.
A replacement reaction occurs when elements switch places in compounds. A single-replacement reaction replaces one element for another in a compound. The metal has to be more active than the cation for the reaction to occur.
108 Single Replacement Reactions M AX MX A Single Replacement. Only a more reactive element. The reactants in a single replacement reaction are an element by itself and a compound.
I have this list of reactions which dont occur. The typical demonstration of this in high school chemistry classes everywhere is to suspend a piece of copper wire in a solution of. In a single replacement reaction one of the reactants is more reactive than the other which.
Why do scientists use an activity series to determine if a Chemists often use a list called anactivity seriesto predict whether a single replacement reaction will occur. The chemical reactions we will explore are a representation of the types of reactions found in each group. This can either be in the form of a single replacement reaction or a double replacement reaction.
A single-displacement reaction also known as single replacement reaction or exchange reaction is a chemical reaction in which one element is replaced by another in a compound. A BC B AC. A double-replacement reaction exchanges the cations or the anions of two ionic compounds.
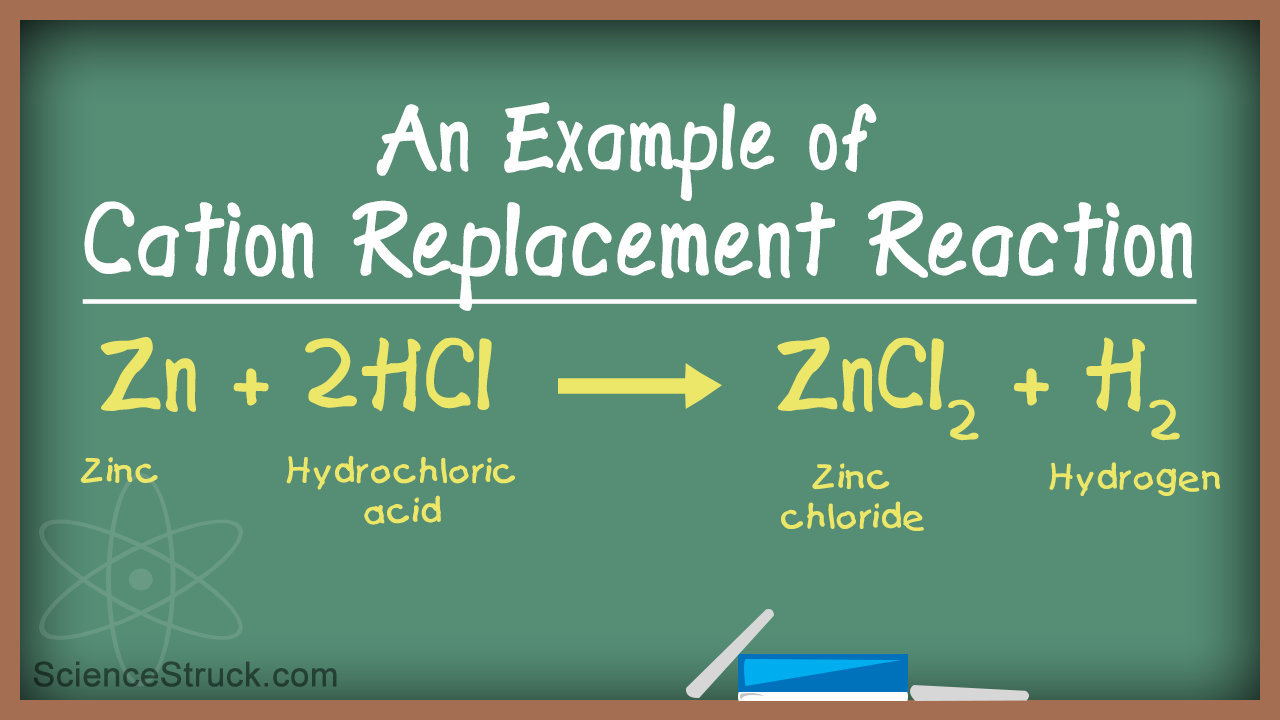
Some metals can replace hydrogen in acids. A single replacement reaction occurs when one element replaces another in a single compound. The periodic table or an activity series can help predict whether single-replacement reactions occur.
There are two types of single replacement reactions. The starting materials are always pure elements such as a pure zinc metal or hydrogen gas plus an aqueous compound. This type of reaction involves ions.
A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound. A single-replacement reaction replaces one element for another in a compound. It can be represented generically as.
The solution is initially blue in color. Uh they do occur. The general pattern of a single replacement reaction is shown below.
Where either and are different metals or any element that forms cation like hydrogen and is an anion. Double replacement reactions also called double displacement exchange or metathesis reactions occur when parts of two ionic compounds are exchanged making two new compounds. An example of a single replacement reaction occurs when potassium K reacts with water H2O.
When a replacement reaction occurs a new aqueous compound and a different pure element will be generated as products. An activity series is a list of elements organized by. Synthesis Reaction Combination Reaction In a synthesis reaction two or more substances combine to form a new compound.
There is a general description of the main reaction types and specific examples provided in the selection boxes. Attach a copy of the activity series list to this journal. A double-replacement reaction exchanges the cations or the anions of two ionic compounds.
A Reaction where a metal replaces a cation in an ionic compound or acid. In normal displacement reactions reactivity plays a large role and sometimes the reaction doesnt even happen. A halogen replaces another halogen that is in solution.
They occur because either the reaction is an acidbase reaction with the formation of an undissociated product often water or because there is an insoluble product precipitate. A single replacement reaction occurs when one element replaces another element in. If reactivity plays a role in this reaction why does it occur.
A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound. Very active metals such as sodium and potassium can replace hydrogen in water. A metal only replaces a metal and a nonmetal only replaces a nonmetal.
In a single replacement reaction also called a single displacement reaction an element reacts with an ionic compound to give a different free element and a different ionic compound. A single displacement reaction is when a single element replaces another element in a compound.

Single Replacement Single Displacement Reaction

Single Replacement Single Displacement Reaction

Single Replacement Reactions And Net Ionic Equations Youtube

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube

Single Replacement Reaction Definition And Examples
Examples Of Single Replacement Reactions Science Struck

Single Displacement Reaction Definition Examples Video Lesson Transcript Study Com
0 comments
Post a Comment